Hearing Aids to Reach Millions With FDA Over-the-Counter Approval
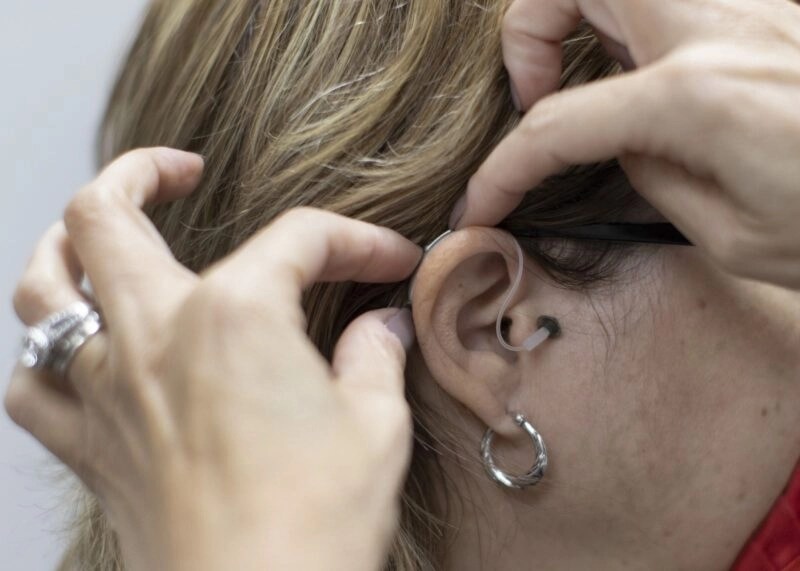
(Bloomberg Law) — Nearly 30 million US adults impacted by hearing loss will now have easier access to hearing aids under a final rule from the FDA allowing them to be purchased over the counter.
The action, which finalizes an October 2021 draft rule, would establish a separate medical device category for certain air-conduction hearing aids used among people 18 years old and older with mild to moderate hearing issues. The new rule stems from President Joe Biden‘s sweeping mandate to crack down on monopolistic practices in agriculture, technology, pharmaceuticals, and other industries. Biden’s order instructed the FDA to take actions within 120 days that would allow hearing aids to be sold over the counter.
“Hearing loss is a critical public health issue that affects the ability of millions of Americans to effectively communicate in their daily social interactions,” FDA Commissioner Robert M. Califf said in a statement. “Establishing this new regulatory category will allow people with perceived mild to moderate hearing loss to have convenient access to a wide array of safe, effective, and affordable purchasing options from their neighborhood store or online.”
The FDA, along with the final rule, issued guidance differentiating between hearing aids and personal sound amplification products, which help people with normal hearing ability amplify sounds in certain environments. Hearing aids are regulated as medical devices by the FDA, unlike sound amplification products, whose manufacturers aren’t subject to product registration requirements.
The 2017 FDA user fee reauthorization included provisions for the FDA to conduct rulemaking to establish an over-the-counter category for hearing aids. The bipartisan legislation also called for the agency to establish safety and effectiveness requirements for products that fall in this category.
Califf said in a call with reporters Tuesday that only about one-fifth of Americans who experience hearing loss seek intervention with the help of hearing aids or other care, due in part to their high costs and the stigma of being perceived as old or disabled. The FDA estimates that the introduction of more over-the-counter hearing aids could lead to roughly $2,800 in savings per pair. Americans currently looking to acquire these products may have to pay up to $5,000 per pair, according to the agency.
In addition to reducing costs for patients, the final rule will “unleash new innovation, which ultimately will mean better quality products and lower prices,” Brian Deese, the White House Director of the National Economic Council, said in Tuesday’s call.
The final rule will take effect in 60 days, and Americans could start seeing over-the-counter hearing aids available at drug and retail stores as soon as mid-October, the FDA said.
Maintaining Safety
The FDA received more than 1,000 comments on the 2021 proposed rule. The Hearing Industries Association told the agency that strict safety and efficacy standards were needed for over-the-counter hearing aids because since 2017, products have entered the market that were “ineffective, of poor quality, and in some cases, dangerous.”
The final rule includes several changes based on the public comments it received, including lowering the maximum sound output of over-the-counter devices to reduce the risk of sound overamplification. It also includes a revised limit to the depth the device can reach in the ear canal, and requires that all hearing aids sold over the counter include a user-adjustable volume control.
The action from FDA is “a significant step forward for the millions of Americans who suffer hearing loss yet are untreated,” HIA President Kate Carr said in a press release. “Hearing loss is unique to each person, and most do not know if their condition is mild, moderate, or greater, caused by another medical issue or something as simple as ear wax.”
“HIA supports the final rule and recommends that the best treatment for hearing loss involves seeing a hearing professional,” Carr added.
The FDA said in its rule that over-the-counter hearing aids will limit cost and medical visit barriers for many, but that it “is not suggesting that licensed persons or their professional services are unimportant or not valuable.”
“We recommend consulting licensed persons in several circumstances, including for the diagnosis of hearing impairment and in the fitting and continued use of OTC hearing aids when consumers choose to seek such services,” the agency said.
Driving Competition
Biden administration officials and policy analysts say the final rule will improve barriers to competition that will benefit both industry and consumers.
Many Americans with mild to moderate hearing impairment have traditionally needed prescriptions from a licensed provider to get a hearing aid, which Deese said Tuesday served as a “barrier to entry” that “kept more companies, or more innovators, from entering the market to compete.”
“That not only limited who could enter the market and get distribution, but the bundling of hearing aids with exams also reduced competition by making it harder for consumers to comparison shop for the best deal,” he added.
Medicare doesn’t cover the costs of hearing aids, or exams for fitting the aids. This has made hearing aids less desirable among many who could benefit from them.
“Hearing aids for the vast majority of Americans are a complete out of pocket expense,” Frank Lin, director of the Johns Hopkins University Cochlear Center for Hearing and Public Health, said in an interview. He added that the FDA in its final rule “struck the right balance” between “fostering safety while ensuring efficacy,” and allowing innovation and competition.
Sens. Elizabeth Warren (D-Mass.) and Chuck Grassley (R-Iowa), who together introduced the Over-the-Counter Hearing Aid Act that Congress passed with user fee reauthorization legislation in 2017, said in a joint statement Tuesday that they’re “thrilled that the FDA has finalized these guidelines.”
“Safe, effective, accessible and affordable hearing aids will now be available over-the-counter for millions of Americans,” they said.
To contact the reporter on this story: Celine Castronuovo at ccastronuovo@bloombergindustry.com
To contact the editors responsible for this story: Cheryl Saenz at csaenz@bloombergindustry.com; Alexis Kramer at akramer@bloomberglaw.com
More stories like this are available on bloomberg.com.
©2022 Bloomberg L.P.