Biden to Order 100 Million More J&J Doses, Boosting Supply
(Bloomberg)
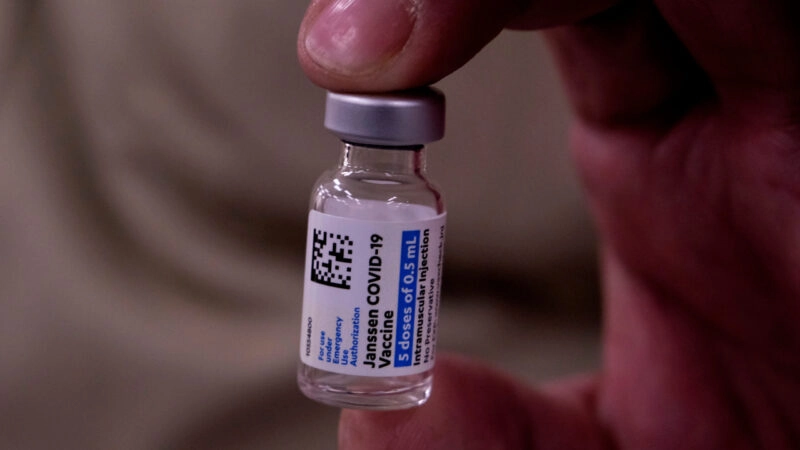
President Joe Biden said doubling the U.S. order of Johnson & Johnson’s one-shot Covid-19 vaccine will help build a stockpile larger than the country’s population, providing backup doses to meet unexpected needs as new variants of the virus emerge.
After the U.S. is satisfied that it has enough supply to meet contingencies it will provide vaccines to other countries, Biden said.
“We’re not going to be ultimately safe until the world is safe”
– Biden said at the White House.
“We’re not going to be ultimately safe until the world is safe,” Biden said at the White House. “And so we’re going to start off making sure Americans are taken care of, first, but we’re then going to try to help the rest of the world.
Biden made the remarks on other countries in response to a question after an event at the White House on Wednesday with J&J Chairman and Chief Executive Officer Alex Gorsky and Merck & Co. chairman and CEO Ken Frazier. The companies last week struck a collaboration to boost production of J&J’s recently authorized Covid-19 vaccine.
The U.S. had previously ordered 100 million doses, which the company has said will be delivered before the end of June. J&J and the government will finalize the new order in coming weeks, officials said.
“I’m doing this because during this wartime effort we need maximum flexibility,” Biden said, explaining the need for the vaccine supply. “A lot can happen, a lot can change, and we need to be prepared.”
Questions Arise
The order is a sign that the U.S. is continuing to stockpile doses as questions arise about which vaccine will be best for children. No shots are authorized for use in people younger than 16, with studies under way on efficacy in older children and others expected to follow on younger ones. J&J will launch adolescent-focused clinical trials in coming weeks.
Health officials also are sounding the alarm over emerging, more-contagious variants of the virus, which could blunt the impact of some inoculations or further spur the need for new rounds of shots.
The initial J&J order, signed in August 2020 under the Trump administration, included an option for as many as an additional 200 million doses. A J&J spokesman said the company remains on track to deliver its first 100 million doses — though it hasn’t publicly committed to delivering all or nearly all of them by May, as Biden has said. J&J has promised them by the end of June.
“The U.S. government has the option to purchase additional doses under a subsequent agreement. We look forward to any future discussions with the U.S. government and to participating in the event at the White House later today,” J&J spokesman Jake Sargent said.
Vaccine Protection
Even as U.S. vaccinations speed up, uncertainty remains. Rochelle Walensky, director of the U.S. Centers for Disease Control and Prevention, said in a briefing Wednesday that health officials are still unsure how long vaccine protection lasts. Anthony Fauci, a Biden Covid adviser who leads the National Institute of Allergy and Infectious Diseases, said Wednesday he expects high school students to be able to be vaccinated this fall, but said studies on safety for several groups are ongoing.
The new J&J doses will be available in the second half of the year, though specific timing will be part of negotiations to finalize the deal, said Biden coronavirus adviser Andy Slavitt. The order follows last week’s announcement of the historic Merck and J&J partnership, which was facilitated by the Biden administration and will materially affect production of the single-shot, easily stored vaccine in the second half of the year.
“He’s doing this because in a wartime effort, which is what we consider this, we need maximum flexibility,” White House press secretary Jen Psaki said. The uncertainties include which vaccine will be best for kids, as well as whether booster shots will be recommended or if variants will require new rounds of inoculations, she said.
“We want to be oversupplied and over-prepared,” she said at Wednesday’s press briefing. “There’s also a chance that we’ll encounter an unexpected challenge on new need in our vaccination efforts, and we are preparing for just that.”
The order was previewed by other moves — the agreement between the companies, and a side deal between Merck and the U.S. government under which it will overhaul some plants to boost vaccine production, including for J&J, and other medicines.
Production Deal
While Biden has credited his administration for facilitating the deal, the companies had been in talks before his inauguration, and those talks accelerated after Jan. 25 when Merck abandoned its own vaccine program. J&J’s shot was authorized about a month later, and the companies’ deal was announced by Biden days after that.
The U.S. is aiding the partnership by investing in Merck’s facilities and using the Defense Production Act, a wartime power, to secure equipment, the official said. The production will eventually be distributed globally and boost global supply, the official said.
With the order, the U.S. is expecting 200 million doses from J&J, which is enough for 200 million people. That’s on top of 300 million doses each from Pfizer Inc. and Moderna Inc., both of which have a two-shot vaccine. Altogether, it’s enough for 500 million people. There are currently about 330 million Americans.