Content Compliance, Simplified
Vodori is on a mission to transform lives by bringing regulated products to market faster. We help life science teams stay compliant and improve the material review process with intuitive, tailored software and peerless customer support. Subscribe for best practices and insights on the material review process, regulatory compliance, and team collaboration.
Content Compliance, Simplified
Amend & Progress
Identifying Key Metrics and Analyzing Data on Performance Gaps Enhance MLR Review Processes
Leveraging data to refine and enhance the promotional material review (MLR) process has become crucial in the ever-evolving landscape of life sciences. Organizations striving for efficiency and compliance need to understand how best to utilize available data to impact their workflow and overall success significantly. How can organizations use data to improve their MLR…
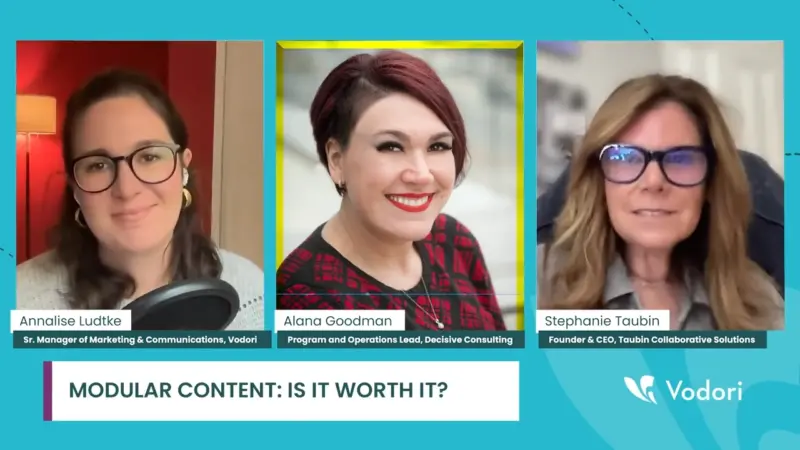
From Content Scramble to Streamlined Success: Demystifying Modular Content in Life Sciences
Modular content is the latest buzz in the life sciences industry, offering a strategic way to streamline content creation and management. With promises of increased efficiency and personalized outreach, many organizations are eager to jump on the bandwagon. But is it truly worth the effort? What exactly is modular content, and how can life…
Department-Specific Reviews can Streamline Material Review Workflows Leading to Faster Approvals
Organizations are increasingly seeking ways to streamline internal processes while ensuring compliance and maintaining high standards. One approach gaining traction is department-specific reviews or role-specific reviews. This method assigns review responsibilities to specific departments or roles, rather than having every piece of content reviewed by all departments. This strategy not only clarifies responsibilities but…
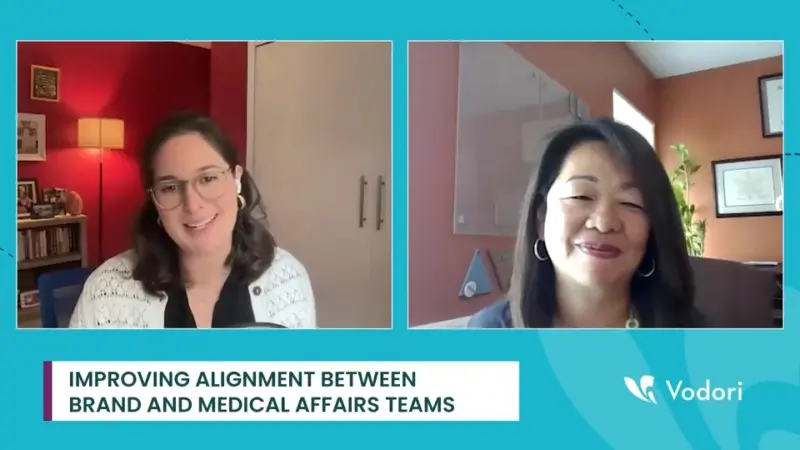
Early Medical Affairs Collaboration Secures Promotional Content Integrity
Strong alignment between brand and medical affairs teams is essential in today’s evolving promotional content landscape. Ensuring seamless collaboration can enhance content quality and efficiency. Effective collaboration between these teams can significantly streamline processes and improve the quality of promotional materials What are the key strategies to foster better collaboration between brand and medical…
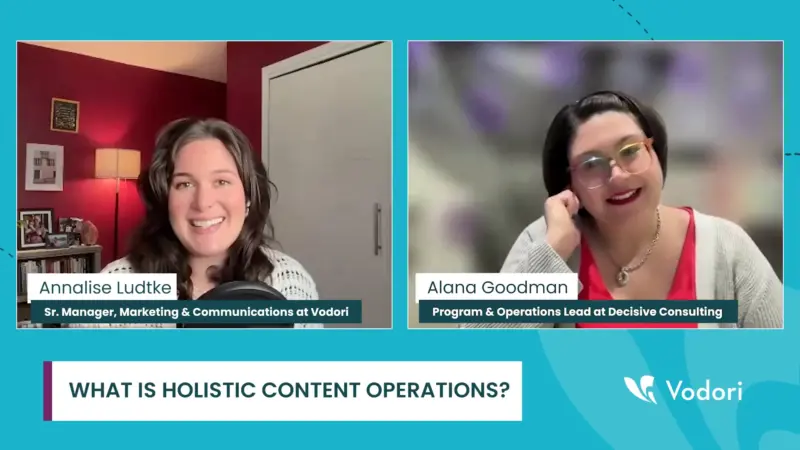
Team Alignment and Clear Objectives Drive Holistic Content Operations in Life Sciences
Understanding holistic content operations is crucial in today’s fast-paced business environment, where effective content management can be the key to success. Holistic content operations encompass the end-to-end processes that ensure content is created, reviewed, and distributed efficiently. A well-structured content operations strategy can significantly enhance organizational workflows and team morale. What exactly constitutes holistic…
Aligning, Appraising, and Simplifying Scientific Data Strengthens Promotional Claims
The pharmaceutical industry faces the challenge of converting complex medical and scientific information into clear, effective promotional claims. This process is critical as it balances the need for accurate scientific communication with the requirement for impactful marketing. The stakes are high, as promotional materials must adhere to stringent regulatory standards while also appealing to…
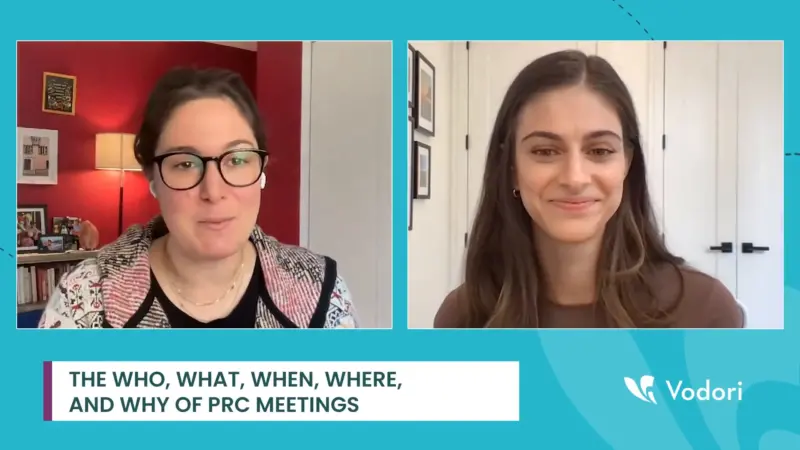
Defining Goals, Leveraging Technology, and Planning Ahead are Essential for Successful Promotional Review Meetings
Today’s fast-paced business environment demands effective review of promotional content. Ensuring all promotional materials are compliant, accurate, and impactful has become crucial. This necessity has given rise to structured promotional review meetings, a trend essential for maintaining quality and compliance in marketing efforts. These meetings facilitate collaboration, ensure adherence to regulatory standards, and help…
AI to Enhance Efficiency and Ensure Content Integrity in the Material Review Process in Life Sciences Sector
The material review process in the life sciences sector is undergoing a significant transformation, thanks to the integration of artificial intelligence (AI). This shift comes at a crucial time when the demand for faster, more accurate reviews is at an all-time high, driven by the rapid pace of medical advancements and regulatory complexities. With…
Strategic Engagement and Clear Communication Optimize Agency Input for Material Review Process Success
Navigating the intricacies of agency relationships is crucial in a world where specialized external partnerships are integral to business operations. The need to effectively manage these collaborations is underscored by the growing dependence on external expertise for tasks ranging from content creation to strategic initiatives. This discussion is vital as businesses aim to optimize…
MLR Review System Ownership Requires Clear Roles, Strong Partnerships, & Data-Driven Decisions
Organizations increasingly depend on sophisticated systems for managing their Material/Legal/Regulatory (MLR) review processes, underscoring the growing importance of system ownership. System reliance raises significant questions about efficiency, compliance, and governance, making it a prime time for a deep dive into the challenges and best practices of system ownership. Understanding who owns these systems and how…
The Missing Piece for MLR Team’s Success is Defining Clear Roles in the Promotional Material Review Process
As businesses increasingly rely on timely and compliant promotional materials, the complexity of managing a promotional material review team has taken center stage. The stakes are exceptionally high in industries such as life sciences, where regulatory compliance is tightly interwoven with market success. Properly managing these teams not only influences the speed of content…
Amend & Progress: Exploring Four Perspectives in 2024 and Beyond
In a dynamic exchange on the Amend & Progress podcast by Vodori, Annalise Ludtke explores four perspectives in 2024 that will help bring effective content to the market now and beyond. With the participation of Vodori’s CEO and Co-Founder, Scott Rovegno, the conversation unveils insights on macro-level topics crucial to the organization’s strategic direction….
Structured Reviews: Great for Elevating Content Quality in Organizational Communication
Digital content is proliferating at an unprecedented rate, and the conversation around content quality has taken center stage. For organizations, particularly those in the life sciences sector, ensuring high-quality content is not just a matter of brand reputation but also of regulatory compliance and effective communication. With the increasing reliance on digital platforms, the…
Revolutionizing Pharmaceutical Ethics: UK’s ABPI Code of Practice Ushers in a New Era of Self-Regulation
In a dynamic era where the intersection of healthcare, regulation, and technology demands constant vigilance, the UK’s ABPI Code of Practice stands as a testament to the pharmaceutical industry’s commitment to self-regulation. Amidst a backdrop of historical challenges and technological advancements, this code serves as the rulebook guiding the ethical conduct of pharma companies…
Revolutionize MLR Team Dynamics with Strategic Meetings and Office Hours
As the professional landscape continues to evolve with the integration of new technologies and methodologies, the emphasis on effective collaboration within Medical, Legal, and Regulatory (MLR) review teams has never been more critical. In an era where team dynamics are frequently reshaped by technological advancements and organizational recalibrations, understanding how to maintain and improve…
The Key to Smooth Audits is Effective Document Management Strategies
In the regulatory landscape, audits serve as essential benchmarks for ensuring organizational compliance and risk mitigation. As regulations grow more intricate, the importance of effectively managing audits has escalated. This timely discussion is crucial as a single oversight can result in considerable financial and reputational harm. So what does it take to turn the…
Ditch the Delays: Set SMART Goals and Conquer Your Promotional Review Process
In the fast-paced marketing world, setting clear, measurable goals for the promotional review process is not just beneficial; it’s essential for efficiency, team morale, and ultimately, success. As businesses navigate the complexities of promotional content approval processes, the beginning of a new year marks a perfect time for reflection and goal setting. How can…
Benchmarks Symposium
Revolutionize Data Analysis: Embrace Median Metrics for Clearer Insights
In the latest episode of Benchmarks Symposium, hosted by Vodori, Annalise Ludtke, the Senior Manager of Marketing and Communications at Vodori, and Jesse Horrell, the VP of Customer Success, embark on an in-depth discussion about the pivotal role of median metrics in enhancing the quality of data analysis presented in Vodori’s 2023 Benchmarks Report….
Pharma Marketing’s New Frontier: Leveraging Content Subtype for Superior and Faster Reviews
Exploring the pivotal aspects of promotional content review in the pharmaceutical industry, the 2023 State of Promotional Review Benchmark Report presented in the Benchmarks Symposium series sheds light on the intricacies of job duration metrics by content subtype. Hosted by Annalise Ludtke, the Senior Manager of Marketing and Communications at Vodori , with the…
Bridging the Gap: Data Insights Level the Playing Field for Promotional Reviews Across Market Segments
Jessy Horrell, the VP of Customer Success at Vodori joins Annalise Ludtke, the Senior Manager of Marketing and Communications on a recent episode of Benchmarks Symposium. The duo took a look into the 2023 State of Promotional Review Benchmarks Report, providing a detailed analysis of promotional review processes across various market segments. The discussion…
Cut Through the Chaos of Review Processes in Healthcare: Implement a Reference Guide for Annotations
In an era where organizations are compelled to do more with less, Vodori’s latest episode of the Benchmarks Symposium sheds light on optimizing promotional review processes. Hosted by Annalise Ludtke, the Senior Manager of Marketing and Communications at Vodori, the video features insights from Elizabeth Calderon, a seasoned Customer Success Manager at Vodori. They…
Don’t Let Promotional Review Processes Slow You Down: Use Data-Driven Strategies for Success
In the rapidly evolving landscape of promotional review processes, Vodori’s 2023 State of Promotional Review Benchmarks Report stands as a critical resource for organizations striving to optimize their processes amidst challenges of limited resources and economic constraints. The latest episode of the Benchmarks Symposium, hosted by Annalise Ludtke, the Senior Manager of Marketing and…
Material Review Agility is the Game Changer for Rapid Compliance in the Pharmaceutical Industry
Material review agility is at the forefront of the latest episode of the Benchmarks Symposium, where Annalise Ludtke, the Senior Manager of Marketing and Communications at Vodori and Anne Swearingen, the CEO at 6 Tangerines Consulting dive into the evolving landscape of promotional review processes within the pharmaceutical industry. They emphasize the importance of…
Unlock Hidden Potential in Healthcare: Data-Driven Benchmarks Reveal Efficiency Gains in Reviews
In an era where the healthcare industry is increasingly data-driven, understanding the nuances of promotional review processes becomes crucial for innovation and efficiency. The Benchmarks Symposium hosted by Vodori provides a deep dive into the state of promotional review benchmarks in 2023. This episode led by Annalise Ludtke, the Senior Manager of Marketing and…